

Baratta, Introduction to Nuclear Engineering, 3d ed., Prentice-Hall, 2001, ISBN: 8-1. Lamarsh, Introduction to Nuclear Reactor Theory, 2nd ed., Addison-Wesley, Reading, MA (1983). Periodic Table Hydrogen 1 H Helium 2 He Lithium 3 Li Beryllium 4 Be Boron 5 B Carbon 6 C Nitrogen 7 N Oxygen 8 O Fluorine 9 F Neon 10 Ne Sodium 11 Na Magnesium 12 Mg Aluminium 13 Al Silicon 14 Si Phosphorus 15 P Sulfur 16 S Chlorine 17 Cl Argon 18 Ar Potassium 19 K Calcium 20 Ca Scandium 21 Sc Titanium 22 Ti Vanadium 23 V Chromium 24 Cr Manganese 25 Mn Iron 26 Fe Cobalt 27 Co Nickel 28 Ni Copper 29 Cu Zinc 30 Zn Gallium 31 Ga Germanium 32 Ge Arsenic 33 As Selenium 34 Se Bromine 35 Br Krypton 36 Kr Rubidium 37 Rb Strontium 38 Sr Yttrium 39 Y Zirconium 40 Zr Niobium 41 Nb Molybdenum 42 Mo Technetium 43 Tc Ruthenium 44 Ru Rhodium 45 Rh Palladium 46 Pd Silver 47 Ag Cadmium 48 Cd Indium 49 In Tin 50 Sn Antimony 51 Sb Tellurium 52 Te Iodine 53 I Xenon 54 Xe Caesium 55 Cs Barium 56 Ba Lanthanum 57 La Hafnium 72 Hf Tantalum 73 Ta Tungsten 74 W Rhenium 75 Re Osmium 76 Os Iridium 77 Ir Platinum 78 Pt Gold 79 Au Mercury 80 Hg Thallium 81 Tl Lead 82 Pb Bismuth 83 Bi Polonium 84 Po Astatine 85 At Radon 86 Rn Francium 87 Fr Radium 88 Ra Actinium 89 Ac Rutherfordium 104 Rf Dubnium 105 Db Seaborgium 106 Sg Bohrium 107 Bh Hassium 108 Hs Meitnerium 109 Mt Darmstadtium 110 Ds Roentgenium 111 Rg Copernicium 112 Cn Nihonium 113 Nh Flerovium 114 Fl Moscovium 115 Mc Livermorium 116 Lv Tennessine 117 Ts Oganesson 118 Og Cerium 58 Ce Praseodymium 59 Pr Neodymium 60 Nd Promethium 61 Pm Samarium 62 Sm Europium 63 Eu Gadolinium 64 Gd Terbium 65 Tb Dysprosium 66 Dy Holmium 67 Ho Erbium 68 Er Thulium 69 Tm Ytterbium 70 Yb Lutetium 71 Lu Thorium 90 Th Protactinium 91 Pa Uranium 92 U Neptunium 93 Np Plutonium 94 Pu Americium 95 Am Curium 96 Cm Berkelium 97 Bk Californium 98 Cf Einsteinium 99 Es Fermium 100 Fm Mendelevium 101 Md Nobelium 102 No Lawrencium 103 Lr
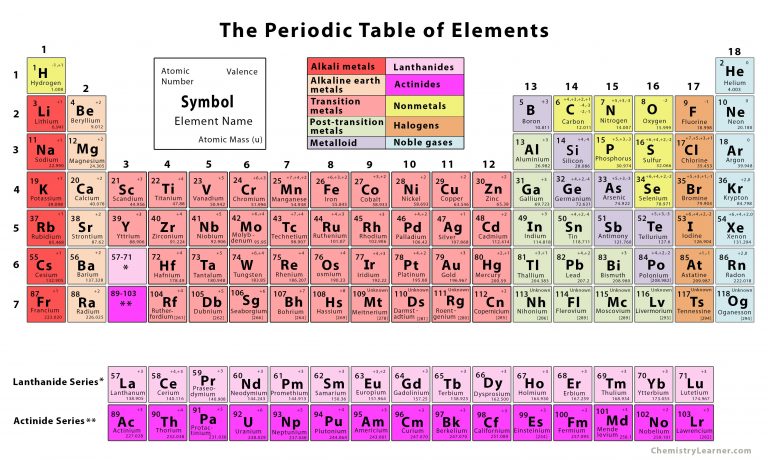
This fact has key implications for the building up of the periodic table of elements. The ordering of the electrons in the ground state of multielectron atoms, starts with the lowest energy state (ground state) and moves progressively from there up the energy scale until each of the atom’s electrons has been assigned a unique set of quantum numbers. It is the Pauli exclusion principle that requires the electrons in an atom to occupy different energy levels instead of them all condensing in the ground state. Each electron is influenced by the electric fields produced by the positive nuclear charge and the other (Z – 1) negative electrons in the atom. The total electrical charge of the nucleus is therefore +Ze, where e (elementary charge) equals to 1,602 x 10 -19 coulombs. The number of electrons in an electrically-neutral atom is the same as the number of protons in the nucleus. The total number of protons in the nucleus of an atom is called the atomic number (or the proton number) of the atom and is given the symbol Z. In the periodic table, the elements are listed in order of increasing atomic number Z.

The number of electrons in each element’s electron shells, particularly the outermost valence shell, is the primary factor in determining its chemical bonding behavior. The configuration of these electrons follows from the principles of quantum mechanics. The chemical properties of the atom are determined by the number of protons, in fact, by number and arrangement of electrons. There is a recurring pattern called the “periodic law” in their properties, in which elements in the same column (group) have similar properties. Generally, within one row (period) the elements are metals to the left, and non-metals to the right, with the elements having similar chemical behaviours placed in the same column.Įvery solid, liquid, gas, and plasma is composed of neutral or ionized atoms. It is organized in order of increasing atomic number. The periodic table is a tabular arrangement of the chemical elements.


 0 kommentar(er)
0 kommentar(er)
